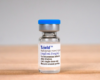
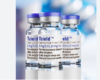
FDA approves Novo Nordisk fast-acting insulin Fiasp
The U.S. Food and Drug Administration on Friday approved Novo Nordisk’s fast-acting insulin to treat diabetes.
The product, known as Fiasp, is designed to help diabetics control post-meal spikes in blood sugar. It is already approved in Canada and Europe.
Fiasp, or faster acting insulin aspart, is designed to work faster than existing fast-acting insulin such as Eli Lilly and Co’s Humalog and Novo Nordisk’s own NovoLog, known as NovoRapid outside the United States.
Last year the FDA declined to approve the product and requested additional information.